Molecular mechanisms of seizure onset in tuberous sclerosis complex.
Tuberous sclerosis complex (TSC) is a genetic disorder that results in abnormal tissue growth in a variety of organs, including the brain. Brain lesions (tubers) occur in 90-95% of TSC patients and contribute to a range of neurological disorders, including epilepsy, autism, and other cognitive and behavioral impairments. Epilepsy afflicts up to 90% of TSC patients, and many TSC patients suffer intractable seizures that do not respond to pharmacological therapy. It is unknown why some tubers cause seizures, while others do not. We are uncovering the molecular mechanisms involved in the onset of seizures associated with TSC tubers. Using tuber tissue that was resected from TSC patients to treat intractable epilepsy we apply advanced genomic and proteomic technologies to characterize gene and protein expression, microRNAs, mutations, and epigenetic modifications that contribute to the epileptogenic potential of cortical tubers. The outcomes of this research are expected to expand our understanding of epilepsy in TSC, identify novel therapeutic targets, and improve diagnostic imaging methods.
This project is funded by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, project R01NS079429.
|
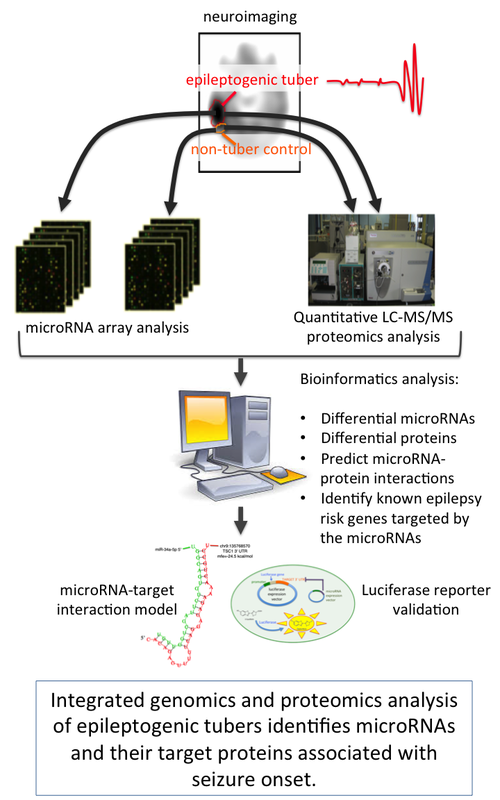
Integration of functional genomics, proteomics, and bioinformatics to identify microRNA activity associated with epilepsy in TSC. Under a research project funded by the National Institute of Neurological Disorders and Stroke (NIH/NINDS), we published the first study to characterize involvement of microRNAs in epilepsy of TSC. MicroRNA's are small RNAs that regulate the expression of target genes and their protein products through degradation of target mRNAs and/or repression of protein translation. They are involved in most cellular processes; however, little was known about microRNAs in TSC. In this study we used tuber tissue involved in seizure onset and adjacent non-tuber control tissue from five TSC patients that had undergone surgery to treat intractable epilepsy. Using microarrays, we first identified microRNAs that had altered expression in epileptogenic (seizure onset) tubers. We then performed quantitative proteomics profiling of the entire proteome to identify protein expression changes in the epileptogenic tissue. Bioinformatics analysis enabled the integration of the two datasets and identification of putative microRNA/protein interactions associated with seizures. Using an external database, we focused on target genes/proteins that are known to be involved with epilepsy. Candidate microRNA/target interactions were computationally modeled, and the interactions were validated using a cell line luciferase reporter assay. Based on our results we developed a mechanistic model of the role of microRNAs and signaling pathways in TSC tuber pathogenesis and epileptogenesis. |